What Is the Importance of the Minor Inorganic Salts
17 What is the importance of the minor inorganic salts to living organisms. POOJA J answered on June 12 2021.

Skin Healing Salve For Eczema Psoriasis 4oz Healing Salves Skin Healing Eczema Psoriasis
It helps transmit and generate nervous impulses and the correct response in muscular stimulus.

. Another importance of salt in the ocean is that it can help to monitor the oxygen intake of ocean living beings. Minor inorganic salts help to maintain the pH of the living organism. Report 0 0 earlier.
While some inorganic compounds are solids with accessible melting points and some are liquids with reasonable boiling points there are not the exhaustive tabulations of meltingboiling point data for inorganic compounds that exist for organics. Melting of ice inflow of river water evaporation rain snowfall wind wave motion and ocean currents that cause horizontal and vertical mixing of the saltwater. Biology 23092019 1700 YRNlightskin3360.
All these salts are found in abundance in the ocean. Mosser List the reasons for variations in salinity in the various oceans. An inorganic compound is a substance that does not contain both carbon and hydrogen.
Thus food starvation increases the salt loss and enhances the deficiency effects. They also act as catalysts in certain biochemical reactions. The reason for variations in salinity in oceans are affected by many factors effecting the various oceans such as.
Impulses acid base water balance cell membrane. These compounds are inorganic. One advantage inorganic fertilizers offer is that they are fast acting.
Simply calcium already represents 2 of our total weight. For example bile salts produced by the liver help break apart dietary fats and calcium phosphate salts form the mineral portion of teeth and bones. This indicates that inorganic salts remain in some sort of chemical combination in the cell.
1 Approved Answer. For the salts of the alkali metals for example both factors are generally unfavourable. In chemistry a salt is a chemical compound consisting of an ionic assembly of positively charged cations and negatively charged anions which results in a compound with no net electric charge.
Such as iodine for the. The more salt means the salinity level will go higher. The component ions in a salt compound can be either inorganic such as.
These nutrient-rich salts dissolve quickly and are immediately available to the. A common example is table salt with positively charged sodium ions and negatively charged chloride ions. Report 0 0 earlier.
Many other salts are important in the body. 4 Ratings 21 Votes In organic salts are sources of essential ions for eg. That is they do not contain both hydrogen and carbon.
During food starvation the animal has to live by burning its own body tissues. Which of the following are examples of dissolved gases found in the ocean. Flocculation begins after the addition of the primary coagulant as the neutralized particles begin colliding and growing in size.
Of the dissolved salts found in seawater _____ are minor inorganic salts or trace72. Most salts consist of calcium phosphorus or magnesium the so-called macrominerals. What is the importance of the minor inorganic salts to living organisms.
And even nitrogen salts. Sodium helps to keep the liquids in your body balanced both inside and outside of your cells according to a study in Advances in Nutrition. A great many inorganic compounds do contain hydrogen atoms.
Important functions include - metabolic processesregulation include transport tofrom cells muscular contraction conduction of nerve. Inorganic compounds essential to human functioning include water salts acids and bases. All living organisms use energy.
The primary difference between organic vs. And higher salinity level will decrease the oxygen intake by ocean living beings and this may resulting their metabolism system to go down. What is another characteristic of all living organisms.
Calc Sulph works to eliminate infections and oozing suppurations including colds sinusitis and can ease skin conditions like boils and acne. Sets found in the same folder. Water is a lubricant and cushion a heat sink a component of liquid mixtures a byproduct of dehydration synthesis reactions and a reactant in hydrolysis reactions.
They also grow and reproduce. Which of the major dissolved salts makes up 116 of these salts. Inorganic compounds is that organic compounds always contain carbon while most inorganic compounds do not contain carbon.
Carbonate is important in blood chemistry and the transfer of energy within a cell. Sodium chlorine potassium etc. Sagittarius November 22-December 21 Salt.
Inorganic compounds are often ionic and so have very high melting points. Note that containing carbon is not sufficient for a compound to be considered organic. Also nearly all organic compounds contain carbon-hydrogen or C-H bonds.
Scorpio governs the reproductive urinary and excretory organs. From what has been said above on the behaviour of the salts that do dissolve in the oxygenated solvents it can be seen that two important factors are the strength with which anions and donor groups like water are held and the lattice energy of the solids. Also the salts of sodium chlorine potassium phosphorus are abundant.
Even if they are inorganic and non-energetic substances the mineral salts intervene therefore in many reactions which are indispensable for our energy functions. Inorganic salts of aluminum or iron are primary coagulants that neutralize the charges on suspended particles. The salts also hydrolyze to form insoluble precipitates that entrap additional particles.
Potassium Chloride is important in heart beat regulation Sodium Chloride is important in maintaining blood pressure. Some of them are freely found in the blood but others are associated with specific biomolecules or also precipitated into solid structures. The salts thus freed diffuse out of the cells and appear in the urine.
What is not an important part of earths second atmosphere. 1 out of 20 points INSTRUCTOR COMMENTS This is true Ashley but can you tell me what these inoganic salts are used for by living organisms in the ocean. Some mineral salts are fundamental constituents of bones and teeth others are necessary for the functioning of enzymes and others are important for the functioning of some organs.
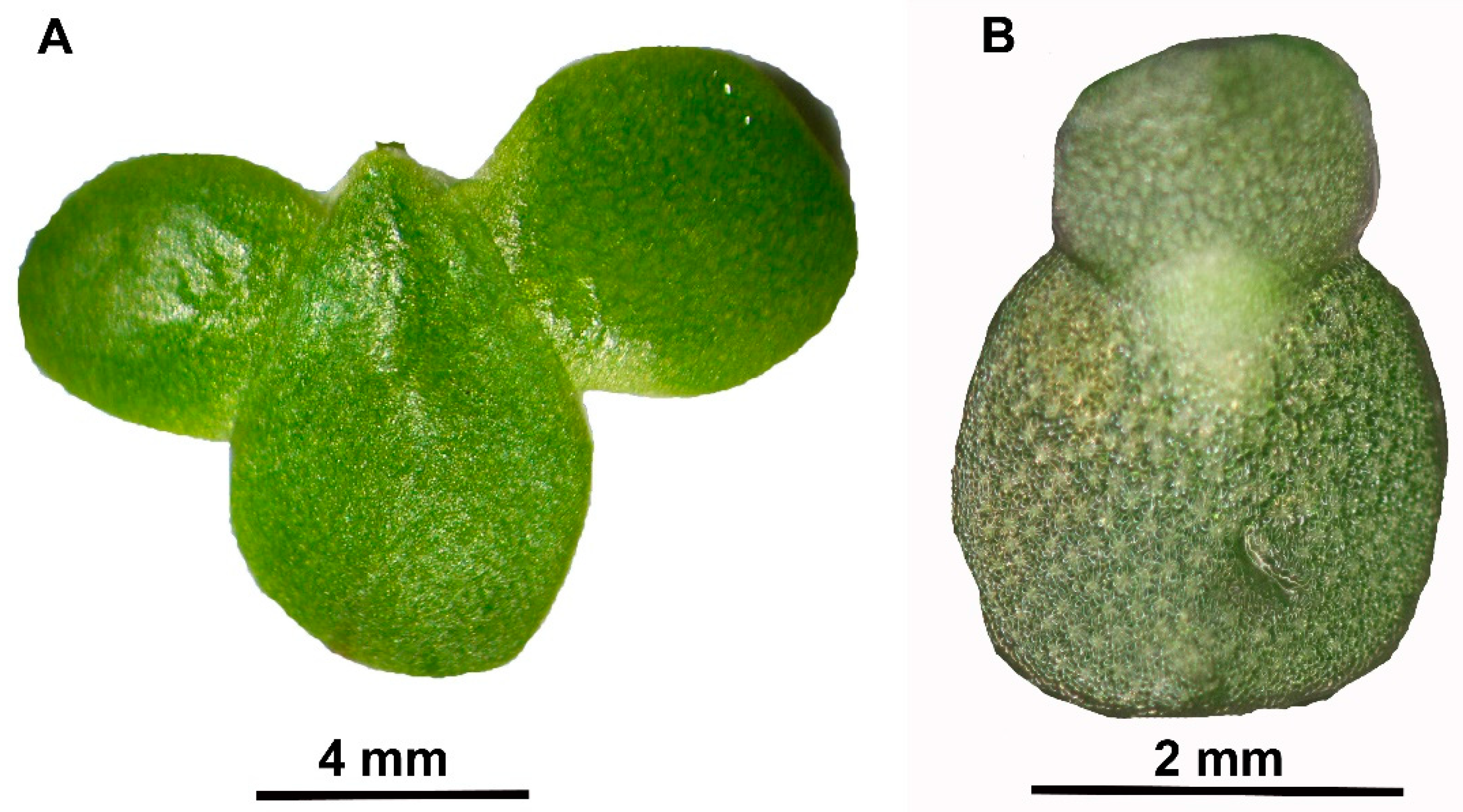
Plants Free Full Text Influence Of The Nitrate N To Ammonium N Ratio On Relative Growth Rate And Crude Protein Content In The Duckweeds Lemna Minor And Wolffiella Hyalina Html

Minor Acidification Of Diafiltration Water Using Various Acidification Agents Affects The Composition And Rennet Coagulation Properties Of The Resulting Microfiltration Casein Concentrate Journal Of Dairy Science
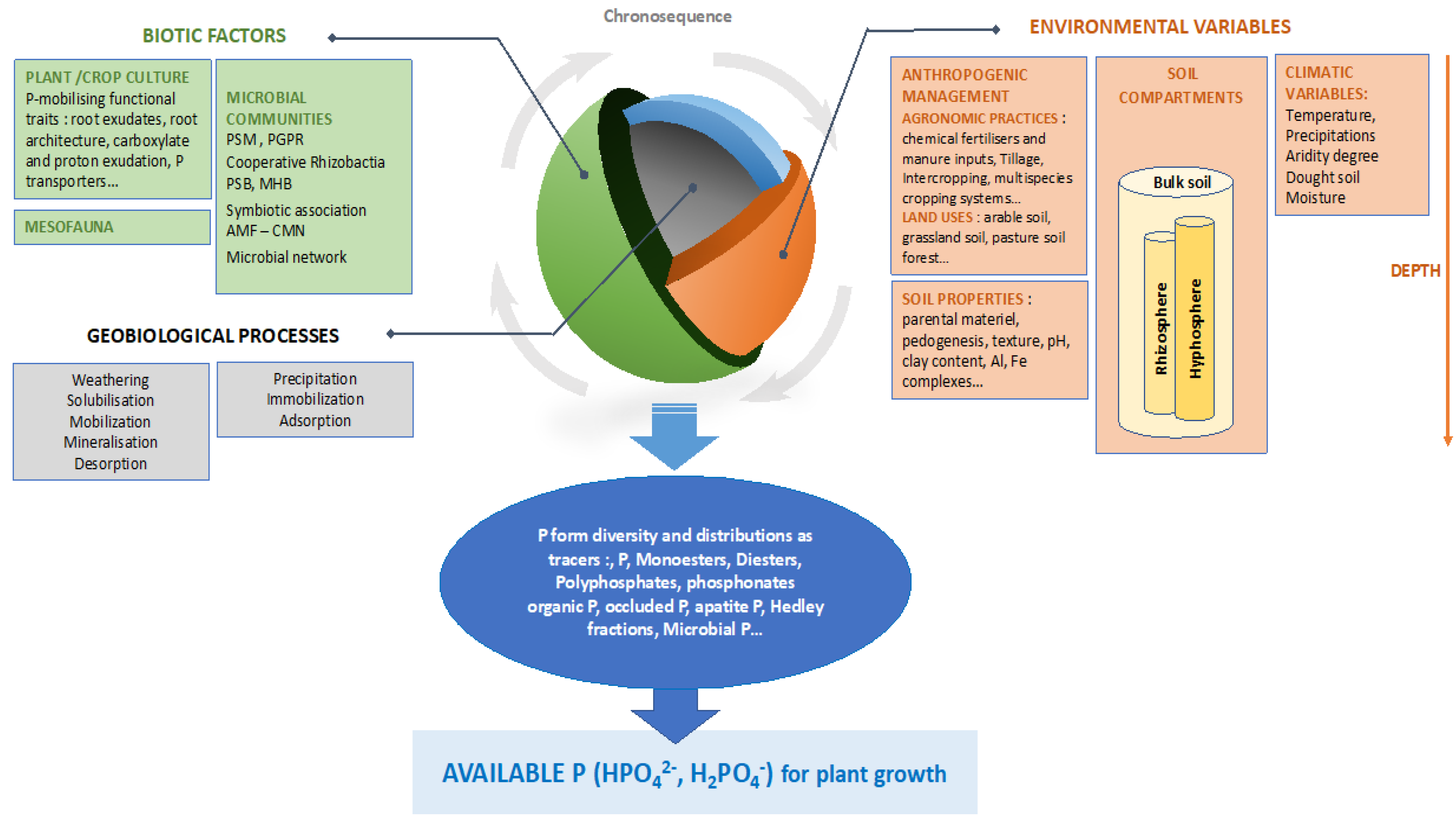
Microorganisms Free Full Text Diversity Of Phosphate Chemical Forms In Soils And Their Contributions On Soil Microbial Community Structure Changes Html
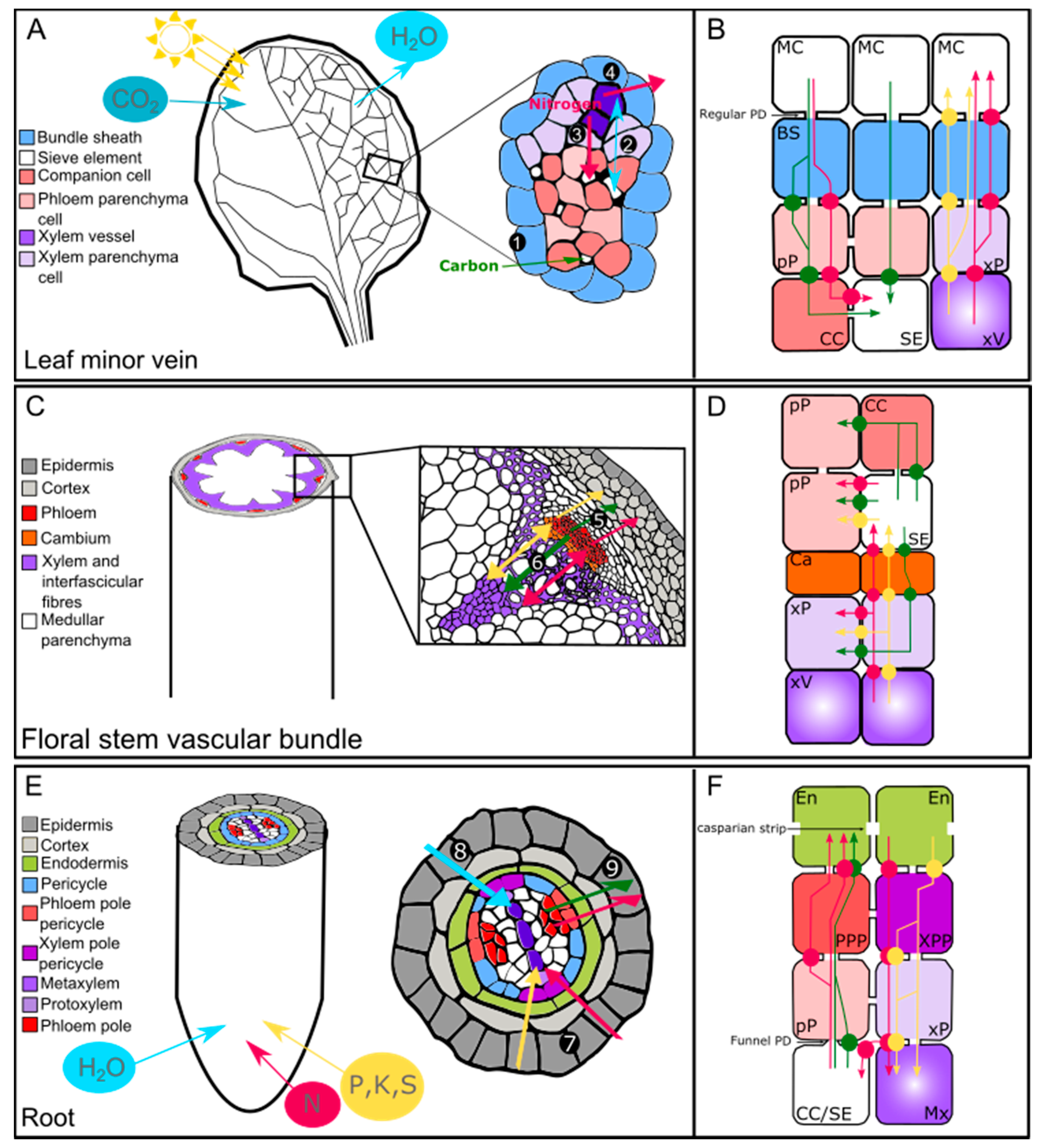
Plants Free Full Text Lateral Transport Of Organic And Inorganic Solutes Html
No comments for "What Is the Importance of the Minor Inorganic Salts"
Post a Comment